Mushroom Body
back
Mushroom body structure
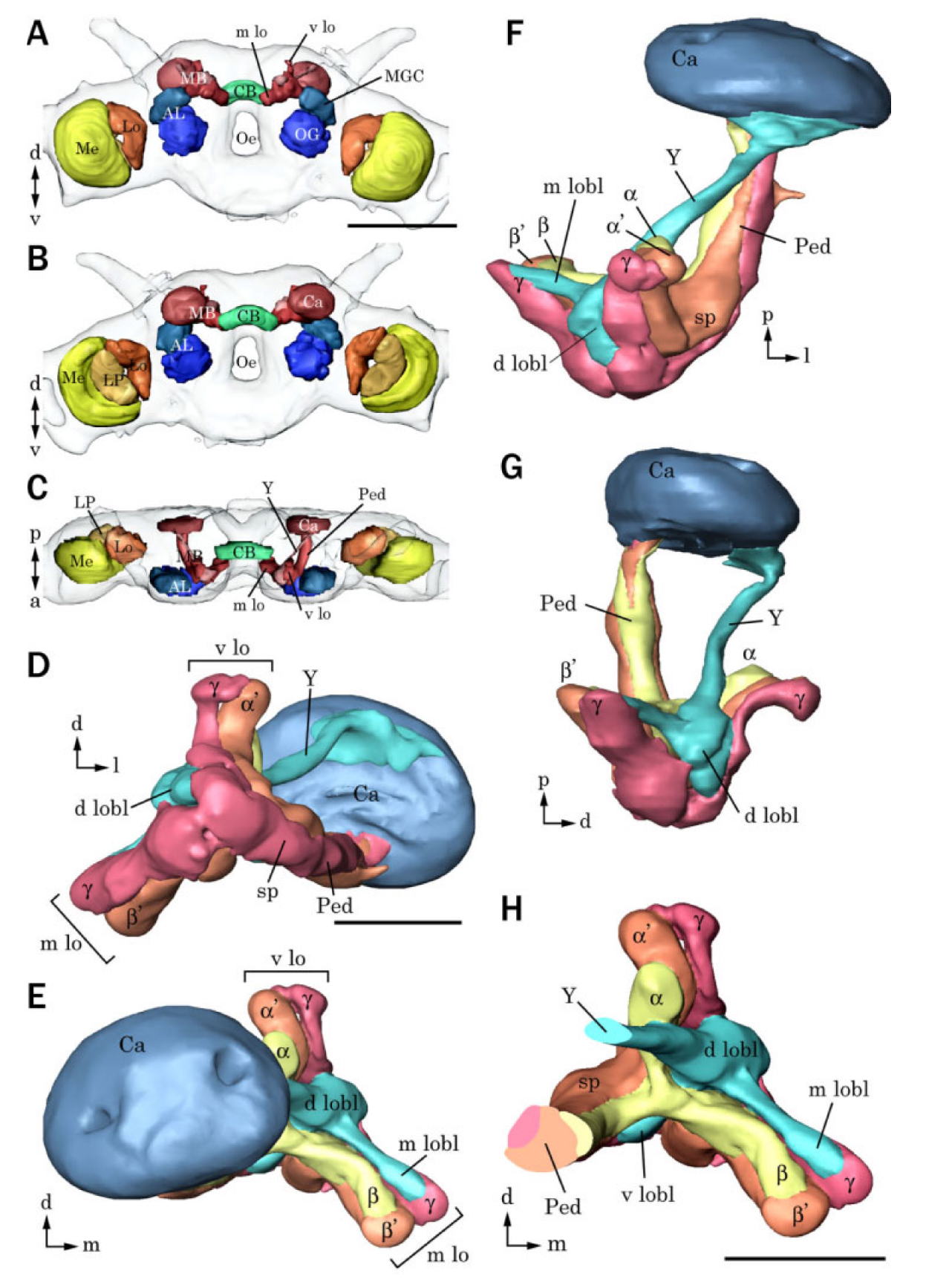
The mushroom body (corpus
pendunculatus) is divided into three major subregions, the calyx,
pendunculus, and the lobes. The calyx is connected to the lobes
though the pedunculus. The lobes are divided into vertical and
medial lobes and lobelet. The vertical lobe is also connected to
the calyx though a separate structure, the Y tract. The calyx
receives input via the antenno-cerebral tracts that connect to
Kenyon cells. Processes of these cells are the major constituent of
the calyces, pendunculi, and lobes. Kenyon cells synapse onto
mushroom body extrinsic neurons, about which little is known in
moths. Groups of Keynon cells form conspicuous units that are
topographically arranged thoughout the pendunculi and lobes.
Mushroom body
function
In the honey bee, retrograde amnesia can
be induced by cooling. This information was used as a base for
cooling experiments of very localised brain structures that have
shown the mushroom bodies to be required for learning success in
classical olfactory conditioning of the proboscis extension
responses [Erber et al., 1980].
Likewise,
the mushroom bodies are involved in spatial learning tasks.
Cockroackes with lesion in the mushroom bodies show performance
deficiencies in spatial learning tasks [Mizunami et al.,
1998].
Extracellular
recording has also revealed a possible role as a premotor control
area. Units were found whose activity was preceeding turning
behaviour during locomotion [Okada et al., 1999].
Mushroom
body neurons
Keynon cells have their dendrites in
the calyx and send axons though the pedunculus and into the lobes.
Their somata are generally very small but still large in moths
compared to most insects. In the cockroach, it has been shown that
the subunits formed by defined groups of Keynon cells also have in
internal structure of repetitive slabs as seen by various classical
staining methods. Each subunit contains to differently staining
slabs As the subdivisions, the slabs run intact though the
pedunculi into the lobes. The slabs are further subdivided into
even thinner sheets, that are also componsed of defined sets of
Keynon cells and may be elementary functional units of the MB.
Extrinsic neurons have been intentified whose dendrites innervate
the pedunculi and beta lobes, making connections only with slabs of
the same staining type. Thus, slabs may be the elementary unit of
mushroom body output [Iwasaki et al., 1999 IS
THIS A JAPANESE PAPER? My info is from Mizunami et al 1998 JCN
399(2):153]. In
Drosophila, it is known from experiments using genetic methods that
aversive olfactory conditioning with electric shocks requires the
function of Kenyon cells [Zars et al., 2000].
Besides
olfactory input, other modalities are also present. This is
well-known because mushroom body output neurons are multimodal in
all species investigated (Homberg and others). to In the bee, it is
believed that a tract from the SOG to the MB calyx carries
gustatory information. In the cockroach, a segregation into input
zones is seen at the level of the calyx, receiving AL PN input,
input from the optic lobes, and from multimodal protocerebral
interneurons [Nishikawa et al 1998; Strausfeld & Li 1999].
Another input is provided by GABAergic calycal giant interneurons
as demonstrated in the cockroach. These cells, which also have
processes in an olfactory area (the lateral horn) are inhibited by
sensory stimuli of various modalities and may thus increase
contrast in the detecting of stimuli by the mushroom body [Nishino
et al., 1998]. In locusts, oscillatory phenomena in
olfactory-induced activity have been intensively investigated and
there is evidence that the mushroom body is optimised to extract
relevant information from the oscillatory output of the antennal
lobe [Perez-Orive et al., 2002].
back
![]()

Reference
Erber
J, Masuhr TH, Menzel R (1980) Localization of short-term memory in
the brain of the bee, Apis mellifera. Physiol Entomol 5:343-358.
Fukushima R, Kanzaki R (2009) Modular subdivision of mushroom bodies by Kenyon cells in the silkmoth. J Comp Neurol 513:315-330.
Mizunami M, Weibrecht JM, Strausfeld NJ (1998) Mushroom bodies of the cockroach: Their participation in place memory. J Comp Neurol 402:520-537.
Okada R, Ikeda J, Mizunami M (1999) Sensory responses and movement-related activities in extrinsic neurons of the cockroach mushroom bodies. J Comp Physiol A 185:115-129.
Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G (2002) Oscillations and sparsening of odor representations in the mushroom body. Science 297:359-365.
Strausfeld NJ, Sinakevitch I, Brown SM, Farris SM (2009) Ground plan of the insect mushroom body: functional and evolutionary implications. J Comp Neurol 513:265-291.
Zars T, Fischer M, Schulz R, Heisenberg M (2000) Localization of a short-term memory in Drosophila. Science 288:672-675.