Methods for examining neurons
back
In the study of the insect brain, the traditional strategy is to assemble
detailed information concerning the constituent neurons that can be integrated
to understand the system level. More recently, imaging techniques, molecular
genetic methods, theory, simulation, and other engineering methods have
been added to electrophysiological recording. For example, experimental
data can be merged to implement a simulation that can be evaluated using
a robot. This section introduces common methods used in the study of insect
nervous systems.
■Intracellular recording and
staining
■(Functional) imaging
■Genetic tools
■Methods
from engineering
■Intracellular recording and staining
First, we
describe the analysis of the morphology of individual neurons. In
the insect brain, the somata from clusters at the surface of the
brain (Figure 1A). For better access, the brain is removed from the
head capsule and immobilised on a glass slide while being immersed
in Ringer's solution. This way, observations can be made with water
immersion lenses (LUMPlanFl/IR, 40x, N.A. 0.80, working distance
3.4 mm or 60x,N.A.
0.90, working distance 2.0 mm) on a fixed stage microscope
(BX51WI,Olympus)
using near-infrared light (>775 nm) to improve visibility. With
such a setup, somata can be visualised by enhanced videomicroscopy
(Figures 1B, C, using a Hamamatsu C2741-79 CCD camera ). Cells were
impaled with a motorised 3D micromanipulator (ONU-31P,Olympus)
using micropipettes(10~100MΩ
impedance).
Pipette
filling solutions contain markers that can be injected into cells
using current. We use the fluorescent dyes Lucifer
Yellow CH,Alexa
Fluor 568 or Neurobiotin(which
can be visualised by binding with Cy-3 conjugated avidin).For
tracing neural circuits, two or even more neurons can be labeled
with different colors. Functional information can be obtained by
labeling for candidate neurotransmitters in addition (Figures 1D,
E).
Impaling
neurons with micropipettes allows both the recording of neural
activity and the identification of neuronal morphology by tracer
injection and subsequent fluorescent detection (Figures 1B, C).
Combining such and other information, a database of neural
structure and function can be established. In the silkmoth, this
approach led to a better understanding of how pheromone information
is processed in the brain and which brain structures are involved
in this process.
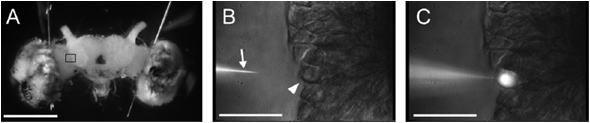
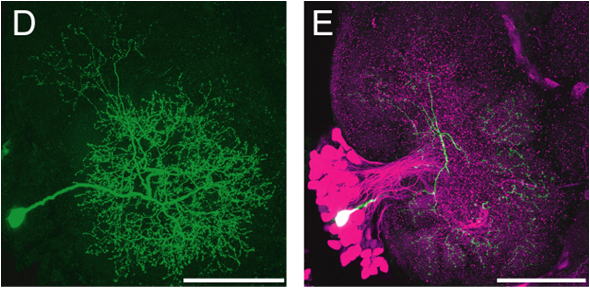
Figure 1: Silkmoth brain and single neurons. (A) Frontal (transversal) view of the silkmoth brain. (B,C) Details of the brain seen in (A) as viewed in differential interference contrast with near-infrared enhanced videomicroscopy (>775nm). Somata can be visualised and it is possible to impale single neurons with a micropipette (arrowhead) mounted on a micromanipulator. Fluorescent dye can be injected into individual neurons. (D) Labeled individual neurons. (E) Neuron labeled in (D, green) in combination with immunohistochemical counterstaining for a neurotransmitter (GABA, in magenta). Scale bars: 1mm (A), 0.1mm (B-E).
back
■Functional imaging
How to investigate the activity of
many neurons simultaneously, that is spatio-temporal patterns of
neural activity? One possibility is to use fluorescent compounds
that allow functional imaging. We focused on the primary olfactory
center of the brain, the antennal
lobe (AL) in order to investigate odorant information
processing. An example of results obtain from such imaging
epxeriment is shown in Figure ?.
A widely used method to
image neural activity is using calcium-sensitive fluorescent
compounds. These can bind calcium ions with some binding constant
and binding goes along with changes in fluorescence intensity.
Calcium concentration in neurons changes in relation to neural
activity, for example by synaptic activity and accompanying action
potentials. It is possible to stain neural tissue with dyes
incorporating an AM (acetoxymethyl) ester moiety by bath
application as these dyes can cross cell membranes but are then
rendered impermeant by intracellular enzymes (esterases). Calcium
changes observed with this very summary method are slow, in the
order of seconds. An example of a calcium response in the antennal
lobe following olfactory stimulation is shown (Figure ?).
More
recently, genetically encoded calcium-sensitive probes have been
designed, for example GCaMP. Such fluorescent proteins can be
introduced into organisms to enable imaging of specific cell
populations. For example, transgenic silkmoths have been produced
in which such probe is expressed in neurons expressing pheromone
rececptor proteins (see below).
Voltage-sensitive dyes
associate with cell membranes and allow the monitoring of
transmembrane voltage (membrane potential) by correlated
fluorescence changes. An advantage is that negative and positive
changes of transmembrane voltage can be recorded, however,
fractional changes in fluorescence intensity are small (in the
order of 0.2% maximally). We have successfully imaged antennal lobe
activation following electrical stimulation of the antennal nerve
and have shown that activation is increased by application of the
neuromodulator serotonin (Fig. ?). Such experiments help to
understand the mechanisms at the base of state-dependent changes in
sensitivity associated with serotonin (5HT) levels in the brain.
back
■Genetic engineering methods
Advances in
large-scale DNA sequencing technology have made it possible to
provide complete genome sequence information for a number of
species. The silk moth genome project is near its completion
[10,11]. Such information is essential to allow the use of
techniques like detection of specific mRNAs by in situ
hybridisation, construct well-specified antigens to generate
antibodies for immunohistochemical methods, and producing
transgenic stains in which for example fluorescent markers are
expressed under the control of chosen promoters.
While in
situ hybridisation and immunohistochemistry can only be applied in
fixed tissued, transgenic animals can be use alive and allow for
visualisation of neurons and many other applications. For instance,
it is possible to genetically control the function of specific
cells, to manipulate excitation and inhibition experimentally in
vivo or to completely knock out some gene of interest. As a result,
genetic approaches are a most important tool in gaining insight
into nervous systems and behaviour today.
The analysis of
an insect central nervous systems using genetic approaches has so
far been carried farthest in the fruit fly(Drosophila
melanogaster)but
in 2000, Tamura has also introduced similar methods to the work
with silkmoths (12). We are currently also using genetic approaches
in our studies of the silkmoth brain.
In the silkmoth, a
transgenic method has been established using the piggyBac
transposon (12). For expression of foreign genes in transgenic
animals, one commonly uses a promoter known to drive the expression
of genes in the target cells (Figure 7A). A reporter gene is also
expressed for confirmation, this is usually a fluorescent protein
such as green fluorescent protein (GFP) or a red fluorescent
protein (DsRed). Using the 3xP3 promoter, photoreceptors were
labeled with DsRed in silkmoth embryos and larvae, for example.
The reporter gene product was relatively evenly distributed
throughout the cytoplasm, facilitating detection.
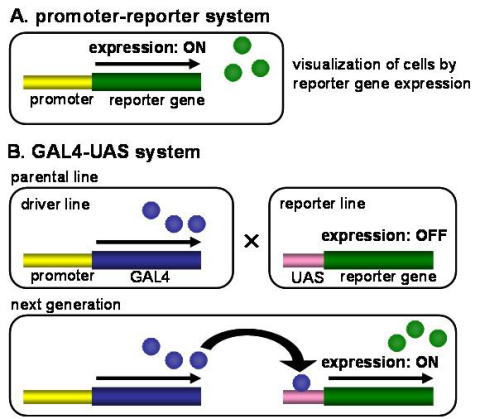
Fig.
7 Schematic diagram of reporter gene expression systems in
transgenic silkmoths. (A) In the promoter-reporter system, promoter
sequence is placed immediately upstream of reporter gene and
directly activates reporter gene expression. (B) In the GAL4-UAS
system, reporter gene expression is activated via GAL4-UAS yeast
transcriptional system.
back
In thefruit fly, signal
sequences determining subcellular localisation of the gene products
have also been added such that specific regions of cells such as
synaptic terminals could be labeled. Such approaches are under
development in the silkmoth, also. Using the genetically encoded
calcium sensor G-CaMP as a reporter gene, it is possible to record
neural activity form the targeted cells. Individuals of transgenic
lines are genetically uniform because they have been generated from
a few individuals initially and are then imbred, thus the same
neurons are labeled in all individuals of a strain.
From
yeast genetics, the transcription activator protein GAL4 and its
promoter target UAS (upstream promoter sequence), have been
introduced to transgenic work in Drosophila. GAL4 is placed under
the control of a promoter for a gene expressed in desired target
cells in one strain whereas a desired reporter gene is placed under
the control of UAS in another strain. As such, both strains are
easy to maintain because only in the GAL4 strain, some GAL4, which
does nothing in flies normally, is produced. Crossing the two
strains combines the information and GAL4 activates the
transcription of the gene under UAS control (Figure 7B). A key
advantage is that UAS lines lines can be crossed with any number of
GAL4 lines in order to express the gene product of interest in
different populations of target cells of choice. Due to the
combinatorial approach, the work required is much less than what
whould be needed if every promoter/gene combination would have to
be produced de novo.
In the silkmoth, the GAL4/UAS system
was first succesfully used by Imamura in 2003 (13). We first
demonstated the use of GAL4/UAS in the silkmoth nervous system
using promoters for two types of peptidergic neurons to express GFP
(Yamagata et al). Thus, labeling brain neurons with genetically
fluorescent proteins is possible in the silkmoth.
In order
to determine the spatial input pattern from the antennae to the
brain upon pheromone stimulation, we used promoters for the odorant
receptors for the major component of the pheromone blend of the
silkmoth, bombykol (receptor gene BmOR114) and the minor component
bombykal (receptor gene BmOR315). Moths were produced in which
these promoters control GAL4 expression and they were crossed with
an UAS-GFP stain for confirmation of the specific target regions of
odorant receptor neurons expressing the two pheromone receptors.
Then, crossed were made with a UAS-GCaMP stain, allowing us to do
calcium imaging in the antennal lobe at the level of the axon
terminals of the odorant receptor neurons (Sakurai et al, in
preparation).
It has thus become possible to apply
molecular genetic tools to brain research in the silkmoth. In the
future, we will employ these tools to specifically ablate neurons,
to alter neurotransmission in specific circuits, and to start
efforts to image neural activity in higher brain areas related to
pheromone information processing. In particular the possibility of
specifically interfering with or controlling neural activity in
known target neurons holds a great promise for progress in
understanding the neural basis of pheromone orientation behaviour
in the silkmoth.
back
■Engineering methods
Useful robots are expected to
become technical systems that should be able to perform tasks of
interest in noisy, naturalistic environments. From another point of
view, they can also be testbeds for models of the generation
mechanisms of insect behaviour. These models in turn can become
useful when they are capable of performing reliably in natural
environments, as insects can do effortlessy. Through appropriate
interfaces, robots and insects can be merged into systems operating
with components of both. This approach is promising for propelling
our understanding of the relation between neural circuits and
behaviour by allowing specific manipulations during behaviour. We
are currently working on three systems in which insect behaviour,
nervous system, and robotics are involved:
・
Robot
control through a silkmoth brain model
・
Insect-steered
robot
・
Robot
control through silkmoth brain activity
back